Percent Yield Calculator
Find the percent yield, actual yield, and theoretical yield of a reaction using the yield calculator below. Simply select which value you want to find.
Percent Yield:
On this page:
How to Calculate Percent Yield
Percent yield is the ratio of the actual yield of a chemical reaction to the theoretical yield, expressed as a percentage.
Because a reaction is rarely 100% efficient, the resulting mass of the product after a reaction is rarely equal to the calculated yield. This occurs when the limiting reagent does not completely react or when other undesirable reactions occur that may form impurities.
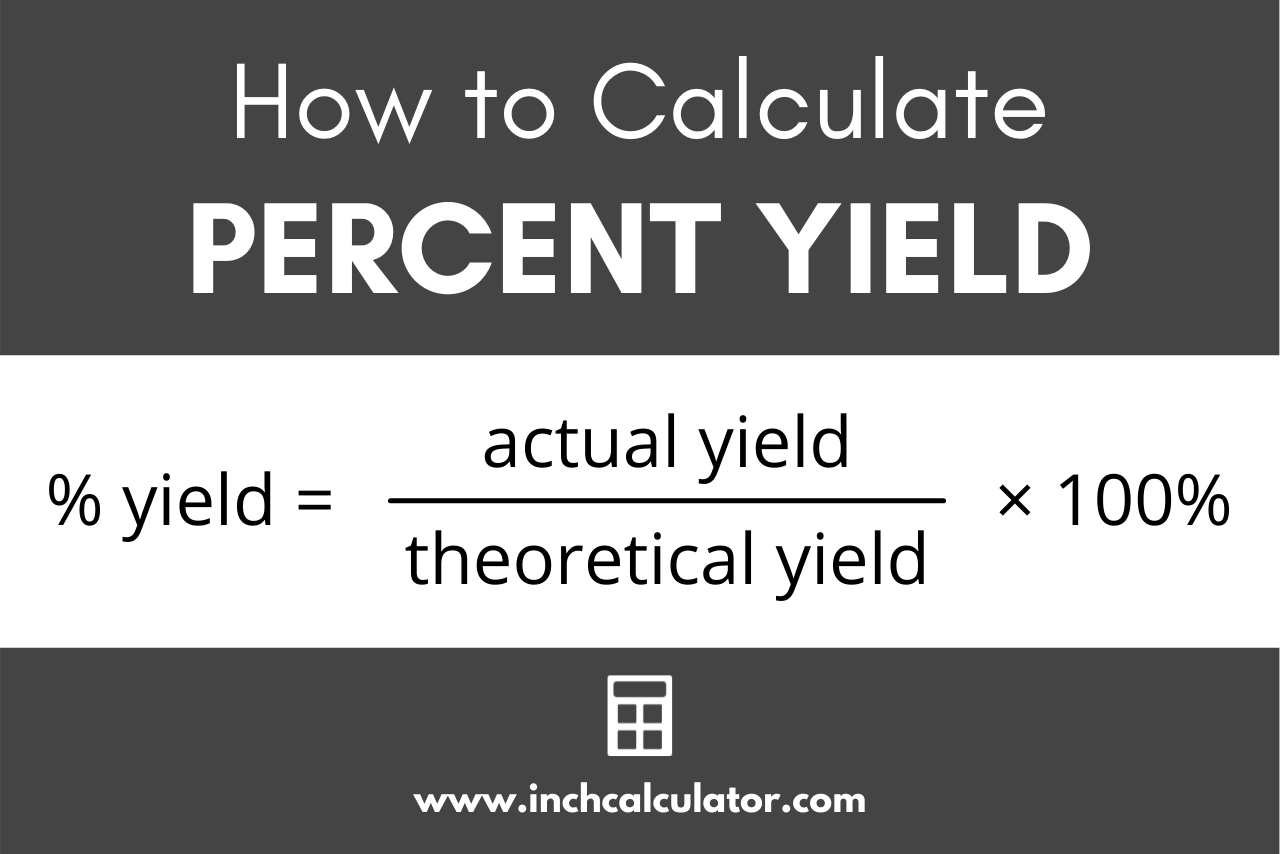
Percent yield is the comparison of the actual yield to the predicted theoretical yield. We can calculate the percent yield using a standard formula.
Percent Yield Formula
Recalling that the percentage yield is the ratio of the actual yield to the theoretical yield, the percent yield formula can be stated as:
percent yield = actual yield / theoretical yield × 100%
Percent yield is equal to the actual mass divided by the theoretical, or calculated mass, multiplied by 100%.[1] Ensure that the inputs in the formula are all in the same unit of mass and convert them if necessary.
Yield calculations can also be carried out in units of moles if that is more convenient. In that case, you can use our grams to moles calculator to convert to mass.
For example, let’s calculate the percent yield of a reaction with a theoretical yield of 4.85 g of product, but an actual yield of 4.47 g.
Apply the formula:
% yield = 4.47 g / 4.85 g × 100%
% yield = 0.922 × 100%
% yield = 92.2%
So, in this example, 92.2% is the percent yield, which is considered excellent.
How to Calculate Percent Loss
The difference between the observed – or actual yield – and the calculated – or theoretical yield – is the percent loss or the percent error. You can calculate the error percentage by using our percent error calculator or the formula below.
Percent Loss Formula
The percent loss formula states:[2]
percent loss = theoretical yield – actual yield / theoretical yield × 100%
The percent loss is equal to the theoretical yield minus the actual yield, divided by the theoretical yield.
For example, let’s calculate the percent loss in the reaction above with a theoretical yield of 4.85 g and an actual yield of 4.47 g.
Apply the formula:
% loss = 4.85 g – 4.47 g / 4.85 g × 100%
% loss = 0.38 g / 4.85 g × 100%
% loss = 0.078 × 100%
% loss = 7.8%
So, in the example above, the percent loss is 7.8%.
A keen observer might have noticed that the percent yield of 92.2% added to the percent loss of 7.8% equals exactly 100%.
So we might simplify the concept of percent loss to be 100% minus the percent yield, or basically the remaining reactant that did not react or formed impurities in the experiment.
Why is Percent Yield Important?
Percent yield is a very important consideration in commercial and industrial chemistry. Since it’s a direct measure of the efficiency of a reaction, it is an important indicator of the amount of reactant required to make the actual amount of product you want.
Therefore, the goal of a chemist working on improving a reaction is most often to improve the yield.
You might be wondering what is considered a good percent yield value. Over 90% yield is considered excellent, 80-90% is deemed very good, 70-80% is good, 50-70% is fair, and below 40% is poor.[3]
Of course, this depends on the actual reaction sequence and how challenging it is to complete.
You can also use our theoretical yield calculator to find the calculated yield of a reaction.
References
- Ramsden, E.N., Calculations for A-level Chemistry, Thornes, 1995, 38.
- LibreTexts, Percent Error, 2021, https://chem.libretexts.org/@go/page/52697
- Furniss, B. S., Hannaford, A. J., Smith, P. W. G., Tatchell, A. R., Vogel's Textbook of Practical Organic Chemistry - Fifth Edition, Longman Scientific & Technical, 1989, 33-34.


