Theoretical Yield Calculator
Calculate the theoretical yield of a reaction given the limiting reagent and desired product.
Theoretical Yield:
Limiting Reagent Moles
Desired Product Stoichiometry
On this page:
- Calculator
- How to Calculate Theoretical Yield
- Theoretical Yield Formula
- Step One: Create a Balanced Equation
- Step Two: Identify the Limiting Reagent
- Step Three: Find the Moles of Limiting Reagent
- Step Four: Apply the Theoretical Yield Formula
- Theoretical Yield vs. Actual Yield or Percent Yield
- References
How to Calculate Theoretical Yield
Theoretical yield is a chemistry term that describes the theoretical maximum product that a chemical reaction can yield. You can calculate the maximum product using a balanced chemical equation and the theoretical yield formula.
Theoretical Yield Formula
You can use the theoretical yield formula to calculate it:
theoretical yield = product molecular weight × limiting reagent moles × product stoichiometry
Thus, the theoretical yield of product is equal to the molecular weight of the product multiplied by the moles of the limiting reagent, multiplied by the stoichiometry of the product.[1]
In order to use the theoretical yield formula, you’ll need to do a few things first.
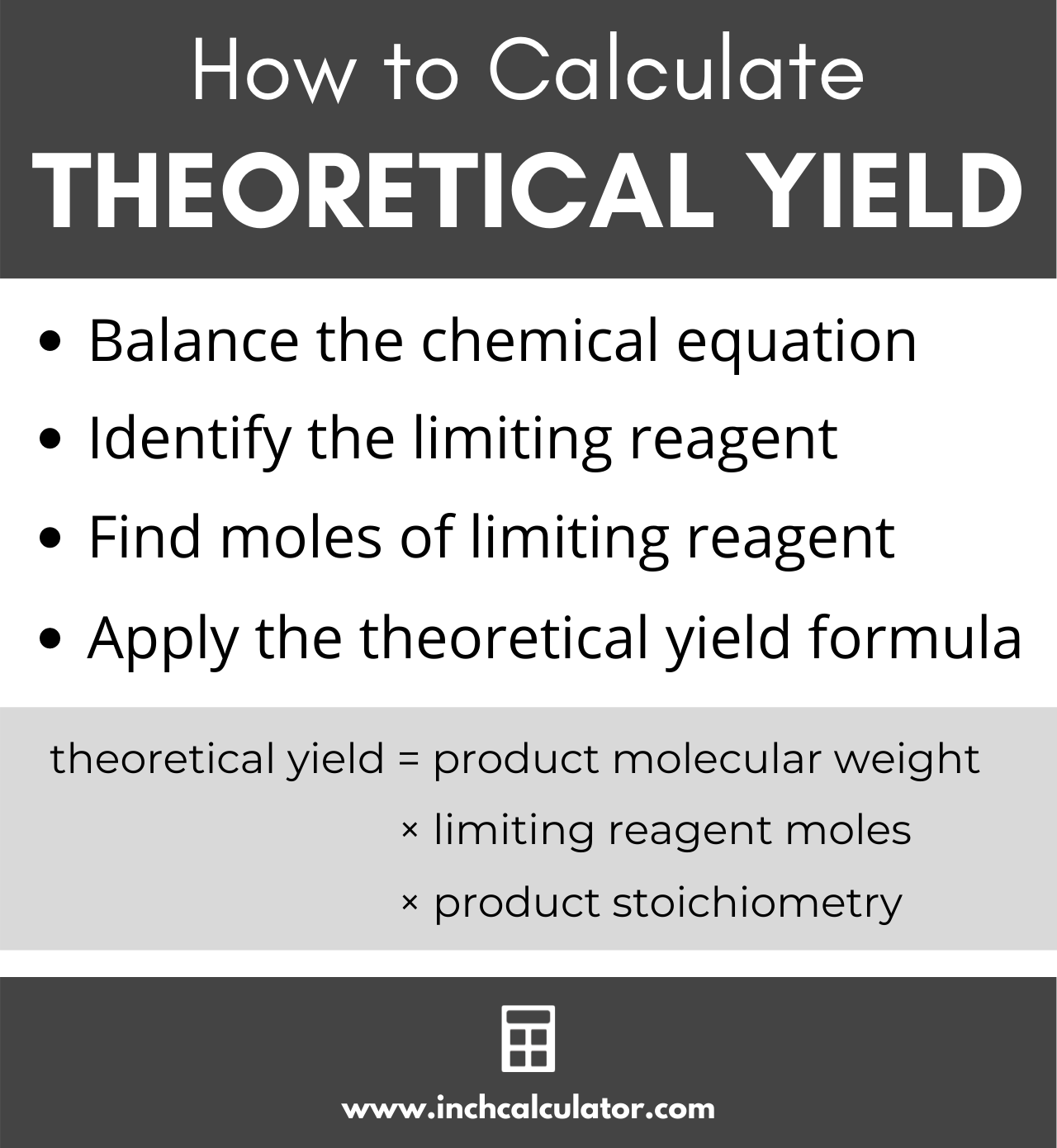
Step One: Create a Balanced Equation
The first step to using the theoretical yield equation is to create a balanced chemical equation. The goal is to create an equation for exactly the same number of atoms of reactants resulting in a desired product with the same number of atoms.
Let’s use an example of balancing the equation of N2 + H2 → NH3. In this example, nitrogen (N2) and hydrogen (H2) will react to create ammonia (NH3).
The equation above is not balanced, however. There are two atoms of nitrogen and two atoms of hydrogen on the left, but there is only one atom of nitrogen and three atoms of hydrogen on the right.
To balance this equation, we’ll need to adjust the coefficients of some of the elements so that there are equal amounts of elements on each side of the equation.[2]
N2 + H2 → NH3
Let’s start with balancing the amount of nitrogen. Since there is one nitrogen atom on the right, add a coefficient of 2 before it to equal the amount of nitrogen atoms on the left.
N2 + H2 → 2NH3
This equation still isn’t balanced as there are now two nitrogen atoms and six hydrogen atoms on the right, but on the left, there are two nitrogen atoms and two hydrogen atoms. But we’re getting closer.
Now, let’s add a coefficient to the hydrogen atoms on the left so that they equal the hydrogen atoms on the right.
N2 + 3H2 → 2NH3
The coefficients in front of each reactant is the reactant’s stoichiometry.
Let’s try an example and balance the following equation:
Fe2O3 + C → Fe CO2
| Fe2O3 + C | → | Fe CO2 |
| Fe = 2 | Fe = 1 | |
| O = 3 | O = 2 | |
| C = 1 | C = 1 |
| 2Fe2O3 + C | → | Fe 3CO2 |
| Fe = 4 | Fe = 1 | |
| O = 6 | O = 6 | |
| C = 1 | C = 3 |
| 2Fe2O3 + 3C | → | 4Fe 3CO2 |
| Fe = 4 | Fe = 4 | |
| O = 6 | O = 6 | |
| C = 3 | C = 3 |
After balancing it, the equation looks like this:
2Fe2O3 + 3C → 4Fe 3CO2
Step Two: Identify the Limiting Reagent
The limiting reagent is the reactant that will be completely reacted before the other reactants. This is necessary to calculate the theoretical yield since the amount of this reactant limits the yield of product.
To find which reactant is the limiting reactant, you need to calculate the moles of each in the reaction. You can do this using the molar mass of each.
Given the moles of each reactant, compare the ratio of moles to the resulting product. The reactant with the highest ratio is the limiting reagent.[3]
Continuing the example above, if 17.25 grams of 2Fe2O3 are reacted with 4.5 grams of 3C, which reactant is the limiting reagent?
Recall the balanced equation:
2Fe2O3 + 3C → 4Fe 3CO2
17.25 grams of 2Fe2O3 = 0.05401 moles
4.5 grams of 3C = 0.1249 moles
Assuming all of the 2Fe2O3 were reacted:
0.05401 moles × 2/3 = 0.0360067 moles of 3C reacted
Since there are 0.1249 moles of 3C available but only 0.0360067 moles will be reacted, there is an excess which means that 3C is the excess reactant and the limiting reactant is 2Fe2O3.
Step Three: Find the Moles of Limiting Reagent
After you have found the limiting reagent, you’ll need to calculate how many moles of the limiting reagent will be in the reaction. You can use the following formula to calculate this:
limiting reagent [moles] = mass / molecular weight × stoichiometry
So, the moles of limiting reagent are equal to the mass of limiting reagent in grams divided by its molecular weight in g/mol, multiplied by the limiting reagent stoichiometry in the reaction, as found above when balancing the equation.
Step Four: Apply the Theoretical Yield Formula
You’re now ready to use the formula above to calculate the theoretical yield.
Let’s continue the example above and calculate the theoretical yield of iron (Fe) given a reaction of 17.25 grams of 2Fe2O3 and 4.5 grams of 3C.
Recall the balanced equation:
2Fe2O3 + 3C → 4Fe 3CO2
We found that 2Fe2O3 was the limiting reagent above, and we know there are 0.05401 moles.
The molecular weight of iron (Fe) is 55.845 g/mol. Feel free to use a molar mass calculator to calculate the molecular weight of your compound.
The stoichiometry of Fe in the balanced equation above is 4.
Let’s put it all together using the theoretical yield formula:
theoretical yield = 55.845 × (0.05401 x 4)
theoretical yield = 12.065 g
Thus, the theoretical yield of iron (Fe) in a reaction of 17.25 grams of 2Fe2O3 and 4.5 grams of 3C is 12.065 g.
Theoretical Yield vs. Actual Yield or Percent Yield
Recall that theoretical yield is the theoretical maximum amount of product that will result from a reaction. However, not all reactions are 100% efficient; in fact, few are.
Actual yield then is the actual amount of product resulting from a chemical reaction.
The comparison between the theoretical yield and the actual yield is the percent yield. So, percent yield is the ratio between the theoretical and actual yields.
You can use a percent yield calculator to calculate this ratio, or you can use the percent yield formula:
percent yield = actual yield / theoretical yield × 100%
Percent yield is equal to the actual yield divided by the theoretical yield, times 100%.
Using this equation it’s also possible to calculate the theoretical yield if the actual yield and percent yield are known.[4]
theoretical yield = actual yield / percent yield × 100%
Theoretical yield is equal to the actual yield divided by the percent yield, multiplied by 100%.
References
- Chapman, B., Jarvis, A., Organic Chemistry, Energetics, Kinetics and Equilibrium, Nelson Thornes, 2003, 60.
- Tuli, G.D., Soni, P.L., The Language of Chemistry or Chemical Equations, S. Chand Publishing, 1977, 25.
- LibreTexts, Limiting Reagents, https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Chemical_Reactions/Limiting_Reagents
- Wink, D.J., Fetzer-Gislason, S., McNicholas, S., The Practice of Chemistry, W. H. Freeman, 2003, 328.

